Trimethylenemethane
[1] Of the three singlet excited states, the first one, 11A1 (1.17 eV above ground), is a closed shell diradical with flat geometry and fully degenerate threefold (D3h) symmetry.
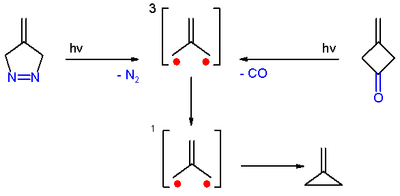
[1] Trimethylenemethane was first obtained from photolysis of the diazo compound 4-methylene-Δ1-pyrazoline with expulsion of nitrogen, in a frozen dilute glassy solution at −196 °C (77 K).
However the product quickly dimerizes to yield 1,4-dimethylenecyclohexane, and also 2-methylpropene by abstracting two hydrogen atoms from other molecules (hydrocarbon or potassium hydride).
[4] A number of organometallic complexes have been prepared, starting with Fe(C4H6)(CO)3, which was obtained by the ring-opening of methylenecyclopropane with diiron nonacarbonyl (Fe2(CO)9).
[5] TMM complexes have been examine for their potential in organic synthesis, specifically in the trimethylenemethane cycloaddition reaction with only modest success.