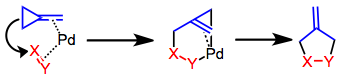
Trimethylenemethane cycloaddition
When electron-rich TMMs are involved, the A orbital serves as the HOMO (leading to fused products if the TMM is cyclic).
When electron-poor (or unsubstituted) TMMs are involved, the S orbital serves as the HOMO (leading to bridged products if the TMM is cyclic).
Three classes of compounds have been used to generate synthetically useful TMM intermediates: diazenes, silyl-substituted allylic acetates and methylenecyclopropenes.
Fused products are generally favored, unless the diazene precursor is substituted with electron-donating groups at the methylene carbon atom.
This reaction is also stereospecific with respect to alkene geometry, and exhibits high selectivity for endo products in most cases.
MCPs lacking stabilizing groups may generate TMM synthons in the presence of palladium(0) or nickel(0) catalysts.
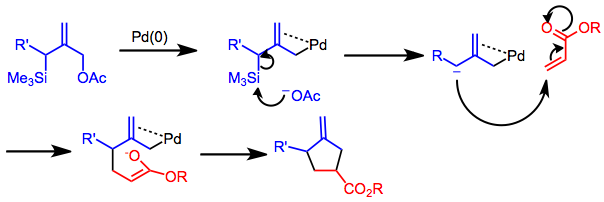
[9] The reaction is highly regioselective, providing only the substitution pattern shown below regardless of the position of the R' group on the starting allylic acetate.
For instance, in the presence of an indium co-catalyst, the reactive 2π component of the cycloaddition below switches from the C-C to the C-O double bond.
Most of these, like TMM cycloaddition, rely on the generation of a suitable three-atom component for combination with a stable two-atom partner such as an alkene or alkyne.
When heated, cyclopropene acetals rearrange to vinylcarbenes, which can serve as the three-atom component in cycloadditions with highly electron-deficient alkenes.
[18] Zinc homoenolates can also serve as indirect three-atom components, and undergo cyclization to cyclopentenones in the presence of an unsaturated ester.
Tetrahydrofuran (THF) at reflux is the most commonly employed solvent system, but photodissociation conditions at low temperature may also be used.
Generally, phosphine or phosphite ligands are required in conjunction with a palladium(0) or nickel(0) source; the most common are Pd2(dba)3 and Ni(cod)2.