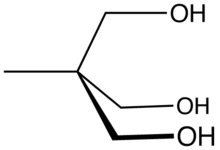
Trimethylolethane
More specifically, it features three primary alcohol groups in a compact neopentyl structure.
More important than TME and closely related is trimethylolpropane (TMP).
Trimethylolethane is produced via a two step process, starting with the condensation reaction of propionaldehyde with formaldehyde: The second step entails a Cannizzaro reaction: A few thousand tons are produced annually in this way.
[1] TME is an intermediate in the production of alkyd and polyester resins, powder coating resins, synthetic lubricants based on polyol esters, stabilizers for plastics, plasticizers, and pigment coatings based on titanium dioxide.
Trimethylolethane based resins have superior weatherability and resistance to alkali and heat.