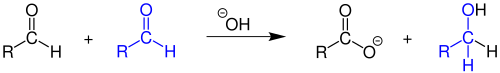
Cannizzaro reaction
More typically, the reaction would be conducted with sodium hydroxide or potassium hydroxide, giving the sodium or potassium carboxylate salt of the carboxylic-acid product: The process is a redox reaction involving transfer of a hydride from one substrate molecule to the other: one aldehyde is oxidized to form the acid, the other is reduced to form the alcohol.
The direct transfer of hydride ion is evident from the observation that the recovered alcohol does not contain any deuterium attached to the α-carbon when the reaction is performed in the presence of D2O.
[5] This can be economically viable if the products can be separated and both have a value; the commercial conversion of furfural into furfuryl alcohol and 2-furoic acid is an example of this.
[6] Alternatively, higher yields of one product (usually the alcohol) can be achieved in the crossed Cannizzaro reaction, in which a sacrificial aldehyde is used in combination with a more valuable chemical.
In this variation, the reductant is formaldehyde, which is oxidized to sodium formate and the other aldehyde chemical is reduced to the alcohol.