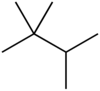
Triptane
It is therefore an alkane, specifically the most compact and heavily branched of the heptane isomers, the only one with a butane (C4) backbone.
It was first synthesized in 1922 by Belgian chemists Georges Chavanne (1875–1941) and B. Lejeune, who called it trimethylisopropylmethane.
[2][3] Due to its high octane rating (112–113 RON, 101 MON[4][5]) triptane was produced on alkylation units starting from 1943[6] for use as an anti-knock additive in gasoline.
It was extensively researched for this role and received the modern name in the late 1930s at a joint laboratory of NACA, National Bureau of Standards, US Army Air Corps and the Bureau of Aeronautics.
[7] As of 2011, it was not a significant component of US automobile gasoline, present only in trace amounts (0.05–0.1%).