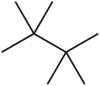
Tetramethylbutane
Because of its highly symmetrical structure, it has a very high melting point and a short liquid range; in fact, it is the smallest saturated acyclic hydrocarbon that appears as a solid at a room temperature of 25 °C.
(Among cyclic hydrocarbons, cubane, C8H8 is even smaller and is also solid at room temperature.)
It is also the most stable C8H18 isomer, with a heat of formation 4.18 kcal/mol (17.5 kJ/mol) lower than that of n-octane, a fact that has been attributed to stabilizing dispersive interactions (electron correlation) between the methyl groups (protobranching).
Despite conditions amenable to the formation of a Grignard reagent, organomagnesium compounds are believed not to be the active species.
[3] The full IUPAC name of the compound is 2,2,3,3-tetramethylbutane, but the numbers are superfluous in this case because there is no other possible arrangement of "tetramethylbutane".