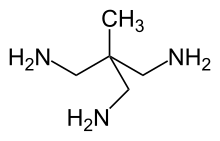
1,1,1-Tris(aminomethyl)ethane
It is classified as a polyamine tripodal ligand, i.e., capable of binding to metal ions through three sites and hence is a tridentate chelating ligand, occupying a face of the coordination polyhedron.
Although azides are potentially explosive, they are excellent and practical source of primary amines.
The required tris(azidomethyl)ethane is obtained from the tritosylate by salt metathesis using sodium azide.
These two steps are:[1] The tripodal TAME ligand coordinates facially to metal ions.
The resulting square planar complex is oxidized with [PtCl6]2− to produce the target Pt(IV) derivatives.