Tris(ethylenediamine)cobalt(III) chloride
The complex was first described by Alfred Werner who isolated this salt as yellow-gold needle-like crystals.
A detailed product analysis of a large-scale synthesis revealed that one minor by-product was [Co(en)2Cl(H2NCH2CH2NH3)]Cl3, which contains a rare monodentate ethylenediamine ligand (protonated).
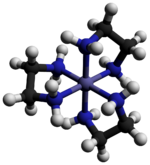
[2] The cation [Co(en)3]3+ is octahedral with Co-N distances in the range 1.947–1.981 Å.
The registry between these ring conformations and the absolute configuration of the metal centers is described by the nomenclature lel (when the en backbone lies parallel with the C3 symmetry axis) or ob (when the en backbone is obverse to this same C3 axis).
[6] Cationic coordination complexes of ammonia and alkyl amines typically crystallize with water in the lattice, and the stoichiometry can depend on the conditions of crystallization and, in the cases of chiral complexes, the optical purity of the cation.