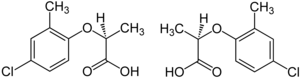
Enantiomer
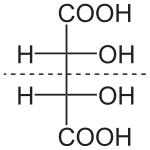
Enantiomer molecules are like right and left hands: one cannot be superposed onto the other without first being converted to its mirror image.
Diastereomers, like enantiomers, share the same molecular formula and are also nonsuperposable onto each other; however, they are not mirror images of each other.
[10] There are three common naming conventions for specifying one of the two enantiomers (the absolute configuration) of a given chiral molecule: the R/S system is based on the geometry of the molecule; the (+)- and (−)- system (also written using the obsolete equivalents d- and l-) is based on its optical rotation properties; and the D/L system is based on the molecule's relationship to enantiomers of glyceraldehyde.
This is the origin of the D/L and R/S notations, and the employment of prefixes levo- and dextro- in common names.
For instance, meso tartaric acid (shown on the right) has two asymmetric carbon atoms, but it does not exhibit enantiomerism because there is a mirror symmetry plane.
The enantiomeric pair of propoxyphene is separately sold by Eli Lilly and company.
One of the partners is dextropropoxyphene, an analgesic agent (Darvon) and the other is called levopropoxyphene, an effective antitussive (Novrad).
[27][28] It is interesting to note that the trade names of the drugs, DARVON and NOVRAD, also reflect the chemical mirror-image relationship.
Or, it may be that both are active, in which case separating the mixture has no objective benefits, but extends the drug's patentability.
[30] In the absence of an effective enantiomeric environment (precursor, chiral catalyst, or kinetic resolution), separation of a racemic mixture into its enantiomeric components is impossible, although certain racemic mixtures spontaneously crystallize in the form of a racemic conglomerate, in which crystals of the enantiomers are physically segregated and may be separated mechanically.
In his pioneering work, Louis Pasteur was able to isolate the isomers of sodium ammonium tartrate because the individual enantiomers crystallize separately from solution.
The second strategy is asymmetric synthesis: the use of various techniques to prepare the desired compound in high enantiomeric excess.
For example, amines with three distinct substituents are chiral, but with few exceptions (e.g. substituted N-chloroaziridines), they rapidly undergo "umbrella inversion" at room temperature, leading to racemization.
However, theoretical physics predicts that due to parity violation of the weak nuclear force (the only force in nature that can "tell left from right"), there is actually a minute difference in energy between enantiomers (on the order of 10−12 eV or 10−10 kJ/mol or less) due to the weak neutral current mechanism.
[17] Throughout this article, "enantiomer" is used only in the chemical sense of compounds of ordinary matter that are not superposable on their mirror image.