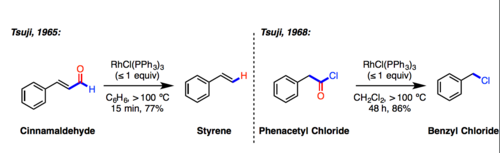
Tsuji–Wilkinson decarbonylation reaction
[1] Strictly speaking, this reaction results in the formation of a rhodium carbonyl complex rather than free carbon monoxide.
The catalytic cycle is assumed to involve oxidative addition of the aldehyde (or acid chloride) to gives a 16e acyl Rh(III)-hydride intermediate, which undergoes migratory extrusion of CO proceed to form an 18-electron d6 Rh(III) carbonyl complex.
In addition to aliphatic, aromatic, and α,β-unsaturated aldehydes, acyl nitriles and 1,2-diketones are also suitable substrates.
In total, the synthesis required 13 steps from commercial starting material, and ~15 mg of [(–)-presilphiperfolan-8-ol] has been prepared with spectral properties and optical rotations matching that of the natural isolate.” Unfortunately, the Tsuji–Wilkinson decarbonylation is stoichiometric.
The product bis(triphenylphosphine)rhodium carbonyl chloride is not readily converted back to a CO-free reagent.