Tumor microenvironment
[2][6][7][8][9] The concept of the tumor microenvironment (TME) dates back to 1863 when Rudolf Virchow established a connection between inflammation and cancer.
However, it was not until 1889 that Stephen Paget's seed and soil theory introduced the important role of TME in cancer metastasis, highlighting the intricate relationship between tumors and their surrounding microenvironment.
[10][12] This viewpoint suggested that certain properties or mutations within cancer cells might dictate their metastatic potential, independent of the surrounding tissue environment.
[10] Isaiah Fidler formulated a complementary hypothesis in the 1970s, where he proposed that while the mechanical aspects of blood flow is important, metastatic colonization specifically targets certain organs, known as organotropism.
However, their cytotoxic activity was found to be lower compared to lymphocytes from distant sites, likely due to the overall immunosuppressive state in tumor-bearing individuals.
VEGF activates the endothelial cells, which begins the process of angiogenesis, where new blood vessels emerge from pre-existing vasculature.
[16] The enhanced permeability and retention effect is the observation that the vasculature of tumors tend to accumulate macromolecules in the blood stream to a greater extent than in normal tissue.
[21] The permeable vasculature allows for easier delivery of therapeutic drugs to the tumor, and the lacking lymphatic vessels contribute to an increased retention.
[5] Periods of mild and acute hypoxia and reoxygenation can lead cancer cells to adapt and grow into more aggressive phenotypes.
[24][4] An increased HIF expression can lead tumor cells to shift their metabolism from aerobic to anaerobic, where they obtain energy through glycolysis.
[25] Cells with an elevated glucose metabolism produce lactate, which decreases the pH in the microenvironment from a neutral and healthy 7.35-7.45 to an acidic 6.3-7.0.
[31][32][11] CAFs are one of the most common components of the tumor stroma and are particularly found in the interstitial spaces of breast, prostate, and pancreatic cancer.
[33][28] They interact with cancer cells by secreting a variety of extracellular matrix components or cell-cell adhesion, which is important in regulating the biological behavior of tumors.
These regulations are particularly important for tumor development and influence cancer cell growth, invasion, inflammation, and angiogenesis.
[35] Studies using proteomic analysis and single-cell RNA sequencing have shed more light on the diverse characteristics of CAFs, revealing distinct and sometimes contradictory functions.
[37][38] In healthy skin, the EMC is composed of various molecules such as collagens, glycoproteins, and glycosaminoglycans that regulate functions and mechanical properties.
This process is called extracellular matrix remodeling and is characterized by changes in protein content and enzymatic activity which influences signal transduction and cell-matrix alterations.
Integrins can sense differences between simple, rigid two-dimensional surfaces and complex, malleable three-dimensional environments, altering cellular signaling accordingly.
These receptors interact with various ECM components and create diverse cellular processes that contribute both to normal physiological functions and pathological conditions like cancer.
[41] While ECM remodeling is tightly regulated under normal physiological conditions, it also modulates many of the tumor cell behaviors associated with cancer progression.
[44][46] Additionally, the restructured ECM and its degradation fragments (matrikines) impacts signaling pathways via cell-surface receptor interactions, leading to dysregulated stromal cell behavior and the emergence of an oncogenic microenvironment.
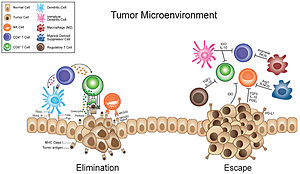
[48] They have the potential to repress T cell responses,[50] can support angiogenesis by producing proteins such as vascular endothelial growth factor (VEGF), and can promote metastasis.
[16] M2-polarized macrophages are, on the other hand, tumor-promoting, because they promote tumor progression by suppressing immunosurveillance,[48] aiding angiogenesis by secreting vascular endothelial growth factor (VEGF)[5] and remodeling the extracellular matrix.
They secrete tumor growth factors, and indirectly support cancer survival by interacting with endothelial cells and carcinoma associated fibroblasts.
Another is the expression of the apoptosis inducer Fas ligand (FasL) in the vasculature of ovarian, colon, prostate, breast, bladder and renal tumors.
[75] Recent research has demonstrated that human germline genetic variants can significantly influence the composition of the tumor microenvironment.
Notably, studies published in the Journal of Clinical Investigation[76] and Nature Communications[77] have highlighted the role of STAT3-enhancing germline mutations and other common genetic variants in modulating the tumor immune landscape and driving therapeutic outcomes.
Strategies included regulation of hypoxia, angiogenesis, cancer-associated fibroblasts (CAFs), extracellular matrix (ECM), and tumor-associated macrophages.
[22][83] Bevacizumab is clinically approved in the US to treat a variety of cancers by targeting VEGF-A, which is produced by both carcinoma associated fibroblasts and tumor-associated macrophages, thus slowing angiogenesis.
CARs are programmed to target tumor-associated antigens as well as replicate rapidly and homogenously, making them potentially very effective as a cancer-therapy.