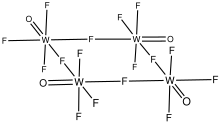
Tungsten oxytetrafluoride
It is usually encountered as product of the partial hydrolysis of tungsten hexafluoride.
In contrast, molybdenum oxytetrafluoride adopts a polymeric structure, although again the fluorides bridge and the oxides are terminal.
[4] It can also be obtained by treating tungsten with a mixture of oxygen and fluorine at high temperatures.
[9] The reaction of tungsten(VI) oxytetrachloride and hydrogen fluoride will also produce WOF4.
[3] WOF4 can also prepared by the reaction of lead(II) fluoride and tungsten trioxide at 700 °C.