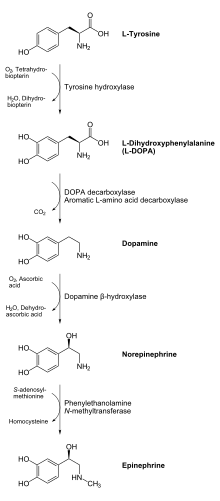
Tyrosine hydroxylase
In humans, tyrosine hydroxylase is encoded by the TH gene,[6] and the enzyme is present in the central nervous system (CNS), peripheral sympathetic neurons and the adrenal medulla.
Under physiological conditions, 4a-BH4 is dehydrated to quinonoid-dihydrobiopterin (q-BH2) by the enzyme pterin-4a-carbinolamine dehydrase (PCD) and a water molecule is released in this reaction.
[11] Since L-DOPA is the precursor for the neurotransmitters dopamine, noradrenaline and adrenaline, tyrosine hydroxylase is therefore found in the cytosol of all cells containing these catecholamines.
This initial reaction catalyzed by tyrosine hydroxylase has been shown to be the rate limiting step in the production of catecholamines.
Tryptophan is a poor substrate for tyrosine hydroxylase, however it can hydroxylate L-phenylalanine to form L-tyrosine and small amounts of 3-hydroxyphenylalanine.
[15] The central ~300 amino acids make up a catalytic core, in which all the residues necessary for catalysis are located, along with a non-covalently bound iron atom.
[19] It has been suggested that this domain might be an intrinsically unstructured protein, which has no clearly defined tertiary structure, but so far no evidence has been presented supporting this claim.
The regulatory domain of tyrosine hydroxylase contains multiple serine (Ser) residues, including Ser8, Ser19, Ser31 and Ser40, that are phosphorylated by a variety of protein kinases.
Clinical features include dystonia that is minimally or nonresponsive to levodopa, extrapyramidal symptoms, ptosis, miosis, and postural hypotension.
[39] Due to the low number of patients and overlapping symptoms with other disorders, early diagnosis and treatment remain challenging.
To provide a basis for improving the understanding of the epidemiology, genotype/phenotype correlation and outcome of these diseases, their impact on the quality of life of patients, and for evaluating diagnostic and therapeutic strategies, a patient registry was established by the noncommercial International Working Group on Neurotransmitter Related Disorders (iNTD).
[41] Furthermore, alterations in the tyrosine hydroxylase enzyme activity may be involved in disorders such as Segawa's dystonia, Parkinson's disease and schizophrenia.
[43] The activity of tyrosine hydroxylase in the brains of patients with Alzheimer's disease has been shown to be significantly reduced compared to healthy individuals.
[45] A consistent abnormality in Parkinson's disease is degeneration of dopaminergic neurons in the substantia nigra, leading to a reduction of striatal dopamine levels.
A direct pathogenetic role of tyrosine hydroxylase has also been suggested, as the enzyme is a source of H2O2 and other reactive oxygen species (ROS), and a target for radical-mediated injury.
This inhibition can lead to a depletion of dopamine and norepinepherine in the brain due to the lack of the precursor L-DOPA (L-3,4-dyhydroxyphenylalanine) which is synthesized by tyrosine hydroxylase.