Ultrasensitivity
This acts to filter out noise, as small stimuli and threshold concentrations of the stimulus (input signal) is necessary for the trigger which allows the system to get activated quickly.
[4] Zero-order ultrasensitivity was first described by Albert Goldbeter and Daniel Koshland, Jr in 1981 in a paper in the Proceedings of the National Academy of Sciences.
[7] For example, in apoptotic process, a model showed that a positive feedback of inhibition of caspase 3 (Casp3) and Casp9 by inhibitors of apoptosis can bring about ultrasensitivity (bistability).
[9] Recently, Jeyeraman et al. have proposed that the phenomenon of ultrasensitivity may be further subdivided into three sub-regimes, separated by sharp stimulus threshold values: OFF, OFF-ON-OFF, and ON.
Based on their model, they proposed that this sub-regime of ultrasensitivity, OFF-ON-OFF, is like a switch-like adaption which can be accomplished by coupling N phosphorylation–dephosphorylation cycles unidirectionally, without any explicit feedback loops.
Recent modeling has shown that multiple phosphorylation sites on membrane proteins could serve to locally saturate enzyme activity.
[23] In 2009, Buchler and Cross constructed a synthetic genetic network that was regulated by protein sequestration of a transcriptional activator by a dominant-negative inhibitor.
Figure 1 in their article illustrates how an active transcription factor can be sequestered by an inhibitor into the inactive complex AB that is unable to bind DNA.
This type of mechanism results in an "all-or-none" response, or ultransensitivy, when the concentration of the regulatory protein increases to the point of depleting the inhibitor.
Robust buffering against a response exists below this concentration threshold, and when it is reached any small increase in input is amplified into a large change in output.
This enhancement in sensitivity of steady state phosphorylated substrate to Km, or the ratio of kinase to phosphatase activity, is termed zero-order to distinguish it from the first order behavior described by Michaelis-Menten dynamics, wherein the steady state concentration responds in a more gradual fashion than the switch-like behavior exhibited in ultrasensitivity.
[17] Additionally, positive feedback can induce bistability in Cyclin B1- by the two regulators Wee1 and Cdc25C, leading to the cell's decision to commit to mitosis.
Assumptions for the allovalency mechanism were based on a general mathematical model that describes the interaction between a polyvalent disordered ligand and a single receptor site[29] It was later found that the ultrasensitivity in Cdk1 levels by degradation of Sic1 is in fact due to a positive feedback loop.
For the case of membrane-bound enzymes acting on membrane-bound substrates with multiple enzymatic sites (such as tyrosine-phosphorylated receptors like the T-Cell receptor), ultrasensitive responses could be seen, crucially dependent on three factors: 1) limited diffusion in the membrane, 2) multiple binding sites on the substrate, and 3) brief enzymatic inactivation following catalysis.
This mechanism of ultrasensitivity is independent of enzyme concentration, however the signal is significantly enhanced depending on the number of binding sites on the substrate.
This mechanism of ultrasensitivity based on local enzyme saturation arises partly from passive properties of slow membrane diffusion, and therefore may be generally applicable.
They found for some ultrasensitive motifs that dynamic range limitations imposed by downstream components can produce effective sensitivities much larger than that of the original module when considered in isolation.
[34] An ultrasensitive switch has been engineered by combining a simple linear signaling protein (N-WASP) with one to five SH3 interaction modules that have autoinhibitory and cooperative properties.
But if the stimulus can regulate localization of multiple components of the signaling cascade, i.e. inhibition of Cdk1-cyclinB1 nuclear export and translocation of the Cdc25C to nucleus, then the outcome is ultrasensitive response, Fig (b).
In fruit flies, three cortical factors have been found to regulate the position of the spindle: heterotrimeric G protein α subunit (Gαi),[45] Partner of Inscuteable (Pins),[46] and Mushroom body defect (Mud).
[48] N-terminal tetratricopeptide repeats (TPRs) in Pins is the binding region for Mud, but is autoinhibited by intrinsic C-terminal GoLoco domains (GLs) in the absence of Gαi.
This intramolecular decoy mechanism allows Pins to establish its threshold and steepness in response to distinct Gαi concentration.
In contrast, long-term potentiation (LTP) occurs when the post-synaptic neuron is subjected to a strong stimulus, and this results in strengthening of the neural synapse (i.e., less neurotransmitter signal is required for activation).
[56] In this way, intracellular calcium can induce a graded, non-ultrasensitive activation of calcineurin at low levels, leading to LTD, whereas the ultrasensitive activation of CaMKII results in a threshold intracellular calcium level that generates a positive feedback loop that amplifies the signal and leads to the opposite cellular outcome: LTP.
Thus, binding of a single substrate to multiple enzymes with different sensitivities facilitates a bistable decision for the cell to undergo LTD or LTP.
[citation needed] It has been suggested that zero-order ultrasensitivity may generate thresholds during development allowing for the conversion of a graded morphogen input to a binary switch-like response.
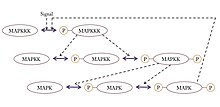
There is paper introducing that engineering synthetic feedback loops using yeast mating mitogen-activated protein (MAP) kinase pathway as a model system.
A ring of 34 FliM proteins around the rotor bind CheY, whose phosphorylation state determines whether the motor rotates in a clockwise or counterclockwise manner.
To transform this graded response system into an ultrasensitive, or switch-like signaling pathway, the investigators created two positive feedback loops.
This finding suggests at least two things: 1) the simplifying assumption that the levels of signaling molecules stay constant in a system can severely limit the understanding of ultrasensitivity's complexity; and 2) it may be possible to induce or inhibit ultrasensitivity artificially by regulating the rates of the entry and exit of signaling molecules occupying a system of interest.