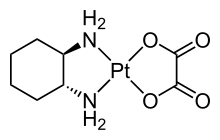
Denticity
In coordination chemistry, denticity (from Latin dentis 'tooth') refers to the number of donor groups in a given ligand that bind to the central metal atom in a coordination complex.
The denticity of a ligand is described with the Greek letter κ ('kappa').
[5][6] Polydentate ligands are chelating agents[7] and classified by their denticity.
Some atoms cannot form the maximum possible number of bonds a ligand could make.
In general, the stability of a metal complex correlates with the denticity of the ligands, which can be attributed to the chelate effect.

monodentate ligands