Virus quantification
It is used in both research and development (R&D) in academic and commercial laboratories as well as in production situations where the quantity of virus at various steps is an important variable that must be monitored.
Other examples of specific instances where viruses need to be quantified include clone screening, multiplicity of infection (MOI) optimization, and adaptation of methods to cell culture.
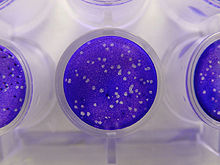
[1] Plaque-based assays are a commonly used method to determine virus concentration in terms of infectious dose.
This assay is based on a microbiological method conducted in petri dishes or multi-well cell culture plates.
Specifically, a confluent monolayer of host cells is infected by applying a sample containing the virus at varying dilutions and then covered with a semi-solid medium, such as agar or carboxymethyl cellulose, to prevent the virus infection from spreading indiscriminately, as would occur in a liquid medium.
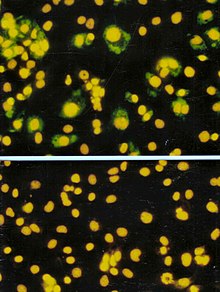
Like the plaque assay, host cell monolayers are infected with various dilutions of the virus sample and allowed to incubate for a relatively brief incubation period (e.g., 24–72 hours) under a semisolid overlay medium that restricts the spread of infectious virus, creating localized clusters (foci) of infected cells.
This endpoint dilution assay quantifies the amount of virus required to kill 50% of infected hosts or to produce a cytopathic effect in 50% of inoculated tissue culture cells.
[citation needed] When used in the context of tissue culture, host cells are plated and serial dilutions of the virus are added.
Most protein-based methods are relatively fast and sensitive[citation needed] but require quality standards for accurate calibration, and quantify protein, not actual virus particle concentrations.
The BCA assay reagent was first developed and made commercially by Pierce Chemical Company (now owned by Thermo Fisher Scientific) which held the patent until 2006.
[17][18] In the BCA assay, a protein's peptide bonds quantitatively reduce Cu2+ to Cu1+, which produces a light blue color.
Enzyme-linked immunosorbent assay (ELISA) is an antibody-based assay that utilizes an antigen-specific antibody chemically linked to an enzyme (or bound to a second antibody linked to an enzyme) to detect the presence of an unknown amount of the antigen (e.g., viral protein) in a sample.
[20] Horseradish peroxidase (HRP) is a common enzyme utilized in ELISA schemes due to its ability to amplify signal and increase assay sensitivity.
[22] Quantitative PCR utilizes polymerase chain reaction chemistry to amplify viral DNA or RNA to produce high enough concentrations for detection and quantification by fluorescence.
In general, quantification by qPCR relies on serial dilutions of standards of known concentration being analyzed in parallel with the unknown samples for calibration and reference.
[23] Sequence-specific probes, such as TaqMan Molecular Beacons, or Scorpion, bind only to the DNA of the appropriate sequence produced during the reaction.
While SYBR Green is easy to use, its lack of specificity and lower sensitivity lead most labs to use probe-based qPCR detection schemes.
[citation needed] There are many variations of qPCR including the comparative threshold method, which allows relative quantification through comparison of Ct values (PCR cycles that show statistically significant increases in the product) from multiple samples that include an internal standard.
In the example of foot-and-mouth disease virus, the ratio of whole virions to RNA copies within an actively replicating host cell is approximately 1:1000.
[26] Advantages of titration by qPCR include quick turnaround time (1–4 hours) and sensitivity (can detect much lower concentration of viruses than other methods).
[27] The technique has the advantage of simultaneously determining the size and concentration, of virus particles in solution with high resolution.
[citation needed] TRPS-bases virus analysis is commercially available through qViro-X systems, which have the ability to be decontaminated chemically by autoclaving after measurement has occurred.
[30] The SP ICP-MS was adapted for the analysis of Single Virus Inductively Coupled Plasma Mass Spectroscopy (SV ICPMS) in a comprehensive study i.e. Degueldre (2021).
With high resolution multi-channel sector field (MC SF) ICP-MS records in SV detection mode, the counting of master and key ions can allow analysis and identification of single viruses.
[citation needed] TEM is a specialized type of microscopy that utilizes a beam of electrons focused with a magnetic field to image a sample.
Sample preparations involve depositing specimens onto a coated TEM grid and negative staining with an electron-opaque liquid.
Sample preparations vary depending on protocol and user but generally require hours to complete.
Because of high instrument cost and the amount of space and support facilities needed, TEM equipment is only available in a few laboratories.