Weak-Link Approach
[1] This method takes advantage of hemilabile ligands -ligands that contain both strong and weak binding moieties- that can coordinate to metal centers and quantitatively assemble into a single condensed ‘closed’ structure (Figure 1).
There are three main components of the WLA methodology that enable the in situ control of supramolecular architecture: 1) the utilization of hemilabile ligands, 2) the choice of metal centers, and 3) the type of allosteric effector.
Typical WLA constructs rely on the allosteric effector’s stronger affinity for the metal center versus the weakly binding Y moiety.
The closed structures can then be reformed in situ by halide abstraction agents, such as noncoordinating silver and thallium salts, or by evacuation of the reaction chamber to remove solvent or small molecules.
[13] The extended structure was successfully obtained by appending secondary terpyridine groups onto the hemilabile ligands within the WLA subunits and allowing them to selectively bind Fe(II) ions (Figure 5).
Allosteric regulation in supramolecular structures generated via the WLA is particularly important in the context of designing and synthesizing novel, bioinspired catalytic systems, where the conformation of the complex controls the activity of the catalyst.
For example, a homologated WLA-based Rh(I) macrocyclic structure has been developed that incorporates pyridine-bisimine Zn(II) moieties and behaves as an efficient and completely reversible allosteric modulator for the hydrolysis of 2-(hydroxypropyl)-p-nitrophenyl phosphate (HPNP), a model substrate for RNA (Figure 7).
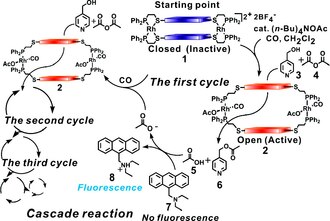
[15] Significantly, the structural changes induced by small molecule regulators Cl− and CO transition this system from a catalytically inactive state to a very active one in a highly reversible fashion.
As in ELISA, the WLA-generated mimic can take a small amount of target (chloride anions) and produce a large fluorescent readout that can be utilized for detection.
By incorporating Zn(II)-salen ligands into a supramolecular assembly, an acyl transfer reaction involving acetic anhydride and pyridylcarbinol as substrates was investigated.
To this end the triple-layer motif was developed, composed of two transition metal nodes, two chemically inert blocking exterior layers, and a single catalytically active interior ligand.
This complex was synthesized using the WLA and halide induced ligand rearrangement processes, and it can be reversibly activated and deactivated through small-molecule or elemental anion effector reactions that assemble and disassemble the trilayer structures.