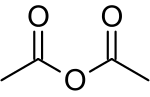
Acetic anhydride
Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis.
It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air.
The internal asymmetry may contribute to acetic anhydride's potent electrophilicity as the asymmetric geometry makes one side of a carbonyl carbon atom more reactive than the other, and in doing so tends to consolidate the electropositivity of a carbonyl carbon atom to one side (see electron density diagram).
Aldehydes react with acetic anhydride in the presence of an acidic catalyst to give geminal diacetates.
[20] As indicated by its organic chemistry, acetic anhydride is mainly used for acetylations leading to commercially significant materials.
[21] It is also used as an active modification agent via autoclave impregnation and subsequent acetylation to make a durable and long-lasting timber.
[23][24] Acetic anhydride is an irritant and combustible liquid; it is highly corrosive to skin and any direct contact will result in severe burns.