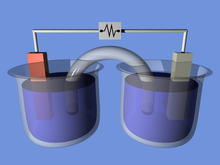
Electric battery
Thus converts high-energy reactants to lower-energy products, and the free-energy difference is delivered to the external circuit as electrical energy.
Benjamin Franklin first used the term "battery" in 1749 when he was doing experiments with electricity using a set of linked Leyden jar capacitors.
[6] This was a stack of copper and zinc plates, separated by brine-soaked paper disks, that could produce a steady current for a considerable length of time.
He thought that his cells were an inexhaustible source of energy,[7] and that the associated corrosion effects at the electrodes were a mere nuisance, rather than an unavoidable consequence of their operation, as Michael Faraday showed in 1834.
[8] Although early batteries were of great value for experimental purposes,[9] in practice their voltages fluctuated and they could not provide a large current for a sustained period.
Near the end of the nineteenth century, the invention of dry cell batteries, which replaced the liquid electrolyte with a paste, made portable electrical devices practical.
Batteries are designed so that the energetically favorable redox reaction can occur only when electrons move through the external part of the circuit.
A voltaic pile can be made from two coins (such as a nickel and a penny) and a piece of paper towel dipped in salt water.
Such a pile generates a very low voltage but, when many are stacked in series, they can replace normal batteries for a short time.
[31] Secondary batteries are not indefinitely rechargeable due to dissipation of the active materials, loss of electrolyte and internal corrosion.
Disposable primary cells cannot be reliably recharged, since the chemical reactions are not easily reversible and active materials may not return to their original forms.
Its low manufacturing cost and its high surge current levels make it common where its capacity (over approximately 10 Ah) is more important than weight and handling issues.
By comparison, the first wet cells were typically fragile glass containers with lead rods hanging from the open top and needed careful handling to avoid spillage.
The remaining space between the electrolyte and carbon cathode is taken up by a second paste consisting of ammonium chloride and manganese dioxide, the latter acting as a depolariser.
[36] Sony has developed a biological battery that generates electricity from sugar in a way that is similar to the processes observed in living organisms.
The VRLA battery uses an immobilized sulfuric acid electrolyte, reducing the chance of leakage and extending shelf life.
Costlier industrial-grade batteries may use chemistries that provide higher power-to-size ratio, have lower self-discharge and hence longer life when not in use, more resistance to leakage and, for example, ability to handle the high temperature and humidity associated with medical autoclave sterilization.
[44] A 4.4 MWh battery system that can deliver 11 MW for 25 minutes stabilizes the output of the Auwahi wind farm in Hawaii.
Internal energy losses and limitations on the rate that ions pass through the electrolyte cause battery efficiency to vary.
Because of internal resistance loss and the chemical processes inside the cells, a battery rarely delivers nameplate rated capacity in only one hour.
Standards for rechargeable batteries generally rate the capacity and charge cycles over a 4-hour (0.25C), 8 hour (0.125C) or longer discharge time.
Types intended for special purposes, such as in a computer uninterruptible power supply, may be rated by manufacturers for discharge periods much less than one hour (1C) but may suffer from limited cycle life.
In contrast to most of today's batteries, the Zamboni pile, invented in 1812, offers a very long service life without refurbishment or recharge, although it can supply very little current (nanoamps).
The Oxford Electric Bell has been ringing almost continuously since 1840 on its original pair of batteries, thought to be Zamboni piles.
[citation needed] Disposable batteries typically lose 8–20% of their original charge per year when stored at room temperature (20–30 °C).
[57] This is known as the "self-discharge" rate, and is due to non-current-producing "side" chemical reactions that occur within the cell even when no load is applied.
However, when "jump starting" a car, the high current can cause the rapid release of large volumes of hydrogen, which can be ignited explosively by a nearby spark, e.g. when disconnecting a jumper cable.
[80][81] The rechargeable battery industry operates nationwide recycling programs in the United States and Canada, with dropoff points at local retailers.
[84] In response to reported accidents and failures, occasionally ignition or explosion, recalls of devices using lithium-ion batteries have become more common in recent years.
[85][86] On 9 December 2022, the European Parliament reached an agreement to force, from 2026, manufacturers to design all electrical appliances sold in the EU (and not used predominantly in wet conditions) so that consumers can easily remove and replace batteries themselves.