William Lipscomb
Independence was encouraged especially in the early years when my mother taught music and when my father's medical practice occupied most of his time."
The young Lipscomb participated in other projects, such as Morse-coded messages over wires and crystal radio sets, with five nearby friends who became physicists, physicians, and an engineer.
His mother questioned his home chemistry hobby only once, when he attempted to isolate a large amount of urea from urine.
Columbia University rejected Lipscomb's application in a letter written by Nobel prizewinner Prof. Harold Urey.
Brief audio clips by Lipscomb about his war work may be found from the External Links section at the bottom of this page, past the References.
In this area Lipscomb proposed that: "... progress in structure determination, for new polyborane species and for substituted boranes and carboranes, would be greatly accelerated if the [boron-11] nuclear magnetic resonance spectra, rather than X-ray diffraction, could be used.
Lipscomb investigated, "... the carboranes, C2B10H12, and the sites of electrophilic attack on these compounds[11] using nuclear magnetic resonance (NMR) spectroscopy.
[12] The calculations provided the first accurate values for the constants that describe the behavior of several types of molecules in magnetic or electric fields.
This study was not successful, in part because the calculation time required for intermetallic compounds was out of reach in the 1960s, but intermediate goals involving boron bonding were achieved, sufficient to be awarded a Nobel Prize.
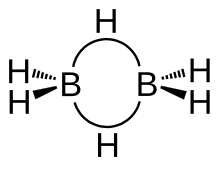
[20] Lipscomb and his graduate students further determined the molecular structure of boranes (compounds of boron and hydrogen) using X-ray crystallography in the 1950s and developed theories to explain their bonds.
Later he applied the same methods to related problems, including the structure of carboranes (compounds of carbon, boron, and hydrogen).
[24] Lipscomb's group also achieved an understanding of it through electron orbital calculations using formulas by Edmiston and Ruedenberg and by Boys.
[25] The Eberhardt, Crawford, and Lipscomb paper[24] discussed above also devised the "styx rule" method to catalog certain kinds of boron-hydride bonding configurations.
[30][31] Calculations by these methods produced accurate Hartree–Fock self-consistent field (SCF) molecular orbitals and were used to study boranes and carboranes.
The ethane barrier to rotation (diagram at left) was first calculated accurately by Pitzer and Lipscomb[32] using the Hartree–Fock (SCF) method.
"[33] "Lipscomb and his coworkers developed the idea of transferability of atomic properties, by which approximate theories for complex molecules are developed from more exact calculations for simpler but chemically related molecules,..."[33] Subsequent Nobel Prize winner Roald Hoffmann was a doctoral student [34] [35] [36] [37] [38] in Lipscomb's laboratory.
Under Lipscomb's direction the Extended Hückel method of molecular orbital calculation was developed by Lawrence Lohr[15] and by Roald Hoffmann.
The 1976 Nobel Prize in Chemistry was awarded to Lipscomb "for his studies on the structure of boranes illuminating problems of chemical bonding".
[47] In a way this continued work on the nature of the chemical bond by his doctoral advisor at the California Institute of Technology, Linus Pauling, who was awarded the 1954 Nobel Prize in Chemistry "for his research into the nature of the chemical bond and its application to the elucidation of the structure of complex substances.
The images below are of Lipscomb's structures from the Protein Data Bank[49] displayed in simplified form with atomic detail suppressed.
Leucine aminopeptidase,[52] (left) a little like carboxypeptidase A, chops off certain amino acids one-by-one from one end of a protein or peptide.
Human interferon beta[54] (left) is released by lymphocytes in response to pathogens to trigger the immune system.
Lipscomb's group also contributed to an understanding of concanavalin A[59] (low resolution structure), glucagon,[60] and carbonic anhydrase[61] (theoretical studies).
[70] Steitz was awarded the 2009 Nobel Prize in Chemistry for determining the even larger structure of the large 50S ribosomal subunit, leading to an understanding of possible medical treatments.
The mineral lipscombite (picture at right) was named after Professor Lipscomb by the mineralogist John Gruner who first made it artificially.
Keeping the crystal cold during data collection produces a less-blurry 3-D electron-density map because the atoms have less thermal motion.