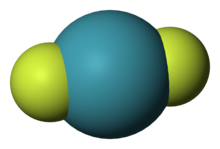
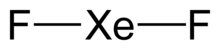Xenon difluoride
Under a pressure of ~50 GPa, XeF2 transforms into a semiconductor consisting of XeF4 units linked in a two-dimensional structure, like graphite.
[13] Shortly after these reports, Weeks, Chernick, and Matheson of Argonne National Laboratory reported the synthesis of XeF2 using an all-nickel system with transparent alumina windows, in which equal parts xenon and fluorine gases react at low pressure upon irradiation by an ultraviolet source to give XeF2.
[14] Williamson reported that the reaction works equally well at atmospheric pressure in a dry Pyrex glass bulb using sunlight as a source.
[15] In the previous syntheses the fluorine gas reactant had been purified to remove hydrogen fluoride.
The unstable organoxenon compound Xe(CF3)2 can be made by irradiating hexafluoroethane to generate CF•3 radicals and passing the gas over XeF2.
The resulting waxy white solid decomposes completely within 4 hours at room temperature.
[20] In the presence of liquid HF, dark green crystals can be precipitated from the green solution at −30 °C: X-ray crystallography indicates that the Xe–Xe bond length in this compound is 309 pm, indicating a very weak bond.
Many such reactions with products of the form [Mx(XeF2)n](AF6)x have been observed, where M can be calcium, strontium, barium, lead, silver, lanthanum, or neodymium and A can be arsenic, antimony or phosphorus.
[24] In 2004, results of synthesis of a solvate where part of cationic centers were coordinated solely by XeF2 fluorine atoms were published.
[34] Xenon difluoride is also used as an isotropic gaseous etchant for silicon, particularly in the production of microelectromechanical systems (MEMS), as first demonstrated in 1995.

2 crystals. 1962.