Zinc carbonate
Zinc carbonate is the inorganic compound with the formula ZnCO3.
It is a white solid that is insoluble in water.
It is prepared by treating cold solutions of zinc sulfate with potassium bicarbonate.
Upon warming, it converts to basic zinc carbonate (Zn5(CO3)2(OH)6).
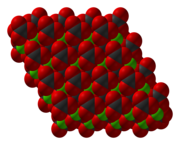
[7] Zinc is octahedral and each carbonate is bonded to six Zn centers such that oxygen atoms are three-coordinate.