Calcium carbonate
It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues.
For example, the formation of aragonite is promoted by the presence of magnesium ions,[21] or by using proteins and peptides derived from biological calcium carbonate.
[14] Organisms, such as molluscs and arthropods, have shown the ability to grow all three crystal polymorphs of calcium carbonate, mainly as protection (shells) and muscle attachments.
[23] Moreover, they exhibit a remarkable capability of phase selection over calcite and aragonite, and some organisms can switch between the two polymorphs.
Industrially important source rocks which are predominantly calcium carbonate include limestone, chalk, marble and travertine.
Eggshells, snail shells and most seashells are predominantly calcium carbonate and can be used as industrial sources of that chemical.
[29][30] Dark green vegetables such as broccoli and kale contain dietarily significant amounts of calcium carbonate, but they are not practical as an industrial source.
The carbonate minerals form the rock types: limestone, chalk, marble, travertine, tufa, and others.
Calcium carbonate contributors, including plankton (such as coccoliths and planktic foraminifera), coralline algae, sponges, brachiopods, echinoderms, bryozoa and mollusks, are typically found in shallow water environments where sunlight and filterable food are more abundant.
Where the oceanic crust is subducted under a continental plate sediments will be carried down to warmer zones in the asthenosphere and lithosphere.
The carbonate is calcined in situ to give calcium oxide, which forms a slag with various impurities present, and separates from the purified iron.
Calcium carbonate is added to swimming pools, as a pH corrector for maintaining alkalinity and offsetting the acidic properties of the disinfectant agent.
[citation needed] Polypropylene compounds are often filled with calcium carbonate to increase rigidity, a requirement that becomes important at high usage temperatures.
[49][50] Calcium carbonate is added to a wide range of trade and do it yourself adhesives, sealants, and decorating fillers.
Ground calcium carbonate is an abrasive (both as scouring powder and as an ingredient of household scouring creams), in particular in its calcite form, which has the relatively low hardness level of 3 on the Mohs scale, and will therefore not scratch glass and most other ceramics, enamel, bronze, iron, and steel, and have a moderate effect on softer metals like aluminium and copper.
A paste made from calcium carbonate and deionized water can be used to clean tarnish on silver.
It may be used as a phosphate binder for the treatment of hyperphosphatemia (primarily in patients with chronic kidney failure).
In 1915, Bertram Sippy introduced the "Sippy regimen" of hourly ingestion of milk and cream, and the gradual addition of eggs and cooked cereal, for 10 days, combined with alkaline powders, which provided symptomatic relief for peptic ulcer disease.
These adverse effects were reversed when the regimen stopped, but it was fatal in some patients with protracted vomiting.
Milk-alkali syndrome declined in men after effective treatments for peptic ulcer disease arose.
Since the 1990s it has been most frequently reported in women taking calcium supplements above the recommended range of 1.2 to 1.5 grams daily, for prevention and treatment of osteoporosis,[59][60] and is exacerbated by dehydration.
Excessive calcium intake can lead to hypercalcemia, complications of which include vomiting, abdominal pain and altered mental status.
Used as an acidity regulator, anticaking agent, stabilizer or color it is approved for usage in the EU,[63] US[64] and Australia and New Zealand.
Several calcium supplement formulations have been documented to contain the chemical element lead,[69] posing a public health concern.
[72] Calcium carbonate is a key ingredient in many household cleaning powders like Comet and is used as a scrubbing agent.
Although his experiment was a success, it did increase the amount of aluminium ions in the area of the brook that was not treated with the limestone.
This shows that CaCO3 can be added to neutralize the effects of acid rain in river ecosystems.
The adjacent table shows the result for [Ca2+] and [H+] (in the form of pH) as a function of ambient partial pressure of CO2 (Ksp = 4.47×10−9 has been taken for the calculation).
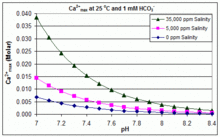
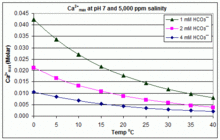
In contrast to the open equilibrium scenario above, many swimming pools are managed by addition of sodium bicarbonate (NaHCO3) to the concentration of about 2 mmol/L as a buffer, then control of pH through use of HCl, NaHSO4, Na2CO3, NaOH or chlorine formulations that are acidic or basic.
Therefore, when HCO−3 concentration is known, the maximum concentration of Ca2+ ions before scaling through CaCO3 precipitation can be predicted from the formula: The solubility product for CaCO3 (Ksp) and the dissociation constants for the dissolved inorganic carbon species (including Ka2) are all substantially affected by temperature and salinity,[83] with the overall effect that [Ca2+]max increases from freshwater to saltwater, and decreases with rising temperature, pH, or added bicarbonate level, as illustrated in the accompanying graphs.