(Pentamethylcyclopentadienyl)aluminium(I)
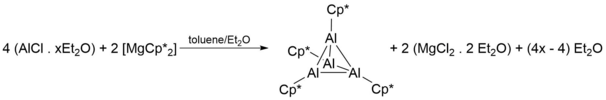
Discovered in 1991 by Dohmeier et al.,[1] AlCp* serves as the first ever documented example of a room temperature stable monovalent aluminium compound.
[1] Additionally, the Al-Al bond in [Cp*Al]4 is significantly shorter than other oligomeric and polymeric Group III M(I)-η5-Cp* compounds such as octahedral [InCp*]6 (394, 336 pm), dimeric [InCp*]2 (363.1 pm), and polymeric [TlCp*] (641 pm), indicating a significantly larger interaction between aluminium atoms in [Cp*Al]4 than monovalent Cp* compounds of In(I) and Tl(I).
NBO calculation of the HOMO-LUMO gap in [Cp*Al] also revealed a significant decreasing in the tetrameric [Cp*Al]4 complex compared to the monomeric [Cp*Al] (4.36 compared to 5.49), which is consistent with density functional theory calculations of analogous systems including superatom complexes of gold, aluminium and gallium.
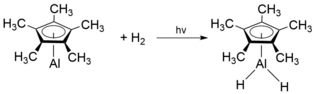
[11] Stabilization of [Cp*Al]4 relative to [CpAl]4 is thought to arise from addition of H-H interactions on the methyl groups attached to the Cp* ligand as opposed to the increased Al-Al bonding interactions.
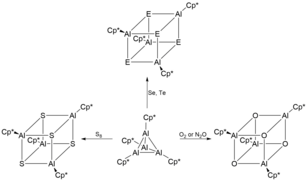
[4] These heterocubane structures are extremely air and moisture sensitive, leading to its decomposition and evolution of H2Se and H2Te respectively.
[13] The resultant iminoalanes was characterized to contain an ideally planar Al2N2 core ring with three coordinate aluminium and nitrogen atoms.
[3] [Cp*Al] is able to act as an atypical exotic ligand in donor-acceptor type bonds.