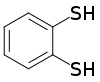
Benzene-1,2-dithiol
Benzene-1,2-dithiol is the organosulfur compound with the formula C6H4(SH)2.
This colourless viscous liquid consists of a benzene ring with a pair of adjacent thiol groups.
The conjugate base of this diprotic compound serves as chelating agent in coordination chemistry and a building block for the synthesis of other organosulfur compounds.
[1] The compound is prepared by ortho-lithiation of benzenethiol using butyl lithium (BuLi) followed by sulfidation:[2] The compound was first prepared from 2-aminobenzenethiol via diazotization.
Ketones and aldehydes condense to give the heterocycles called dithianes: 3,4-Toluenedithiol, also called dimercaptotoluene (CAS#496-74-2), behaves similarly to 1,2-benzenedithiol but is a solid at ambient temperatures (m.p. 135-137 °C).