Metal dithiolene complex
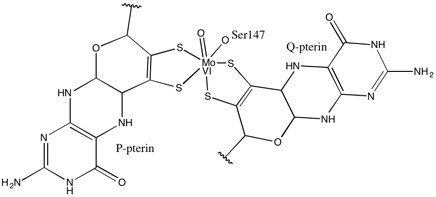
[3] Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin cofactor bound to the Mo or W.[4] Dithiolene metal complexes have been studied since the 1960s when they were first popularized by Gerhard N. Schrauzer and Volker P. Mayweg, who prepared nickel bis(stilbene-1,2-dithiolate) (Ni(S2C2Ph2)2) by the reaction of nickel sulfide and diphenylacetylene.
The substituents on the backbone of the dithiolene ligand, R and R', affect the properties of the resulting metal complex in the expected way.
Reflecting the impossibility to provide an unequivocal description of the structure, McCleverty introduced the term 'dithiolene' to give a general name for the ligand that does not specify a particular oxidation state.
It was proposed to use dithiolene metal complexes that bind unsaturated hydrocarbons at the sulfur centers for industrial olefin (alkene) purifications.
Common alkenedithiolates are 1,3-dithiole-2-thione-4,5-dithiolate[11] and maleonitriledithiolate (mnt2−):[12] Some alkenedithiolates are generated in situ, often by complex organic reactions: Once generated, these anions are deployed as ligands: Often the initially formed, electron-rich complex undergoes spontaneous air-oxidation: An early and still powerful method for the synthesis of dithiolenes entails the reaction of α-hydroxyketones, acyloins, with P4S10 followed by hydrolysis and treatment of the mixture with metal salts.
This electrophilic reagent oxidatively adds to many low valent metals to give bis- and tris(dithiolene) complexes.
More modern versions of this method entail the reaction of electrophilic acetylenes such as dimethyl acetylenedicarboxylate with well defined polysulfido complexes.
Early studies on dithiolene ligands, although not called by that name until the 1960s,[14]: 58 [15] focused on the quinoxaline-2,3-dithiolates and 3,4-toluenedithiolates, which form brightly colored precipitates with several metal centres.
Dithiolenes lacking benzene backbones represented an important development of the area, especially maleonitrile-1,2-dithiolate ("mnt"), (NC)2C2S2−2, and ethylenedithiolene, H2C2S2−2.