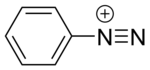
Diazonium compound
The linear free energy constants σm and σp indicate that the diazonium group is strongly electron-withdrawing.
Thus, the diazonio-substituted phenols and benzoic acids have greatly reduced pKa values compared to their unsubstituted counterparts.
In other words, the diazonium group raises the ionization constant Ka (enhances the acidity) by a million-fold.
However, one can isolate diazonium compounds as tetrafluoroborate or tosylate salts,[8] which are stable solids at room temperature.
When the coupling partners are arenes such as anilines and phenols, the process is an example of electrophilic aromatic substitution: The deep colors of the dyes reflects their extended conjugation.
[23] Arenediazonium cations reduced by hypophosphorous acid,[24] ethanol,[25] sodium stannite[26] or alkaline sodium thiosulphate[27] gives benzene: An alternative way suggested by Baeyer & Pfitzinger is to replace the diazo group with H is: first to convert it into hydrazine by treating with SnCl2 then to oxidize it into hydrocarbon by boiling with cupric sulphate solution.
In contrast, Fu reported the trifluoromethylation using Umemoto's reagent (S-trifluoromethyldibenzothiophenium tetrafluoroborate) and Cu powder (Gattermann-type conditions).
Treatment of benzenediazonium chloride with potassium ethylxanthate followed by hydrolysis of the intermediate xanthate ester gives thiophenol: The aryl group can be coupled to another using arenediazonium salts.
[34] Alternatively similar borylation can be achieved using transition metal carbonyl complexes including dimanganese decacarbonyl.
[37] Benzenediazonium chloride reacts with compounds containing activated double bonds to produce phenylated products.
[39] In a potential application in nanotechnology, the diazonium salts 4-chlorobenzenediazonium tetrafluoroborate very efficiently functionalizes single wall nanotubes.
These added substituents prevent the tubes from forming intimate bundles due to large cohesive forces between them, which is a recurring problem in nanotube technology.
In one study, the silicon surface is washed with ammonium hydrogen fluoride leaving it covered with silicon–hydrogen bonds (hydride passivation).
[41] The reaction of the surface with a solution of diazonium salt in acetonitrile for 2 hours in the dark is a spontaneous process through a free radical mechanism:[42] So far grafting of diazonium salts on metals has been accomplished on iron, cobalt, nickel, platinum, palladium, zinc, copper and gold surfaces.
An in silico study [45] demonstrates that in the period 4 elements from titanium to copper the binding energy decreases from left to right because the number of d-electrons increases.
The use of sodium dithionite is an improvement over stannous chloride since it is a cheaper reducing agent with fewer environmental problems.
Alkanediazonium ions, otherwise rarely encountered in organic chemistry, are implicated as the causative agents in the carcinogens.