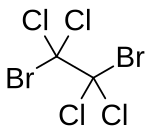
1,2-Dibromotetrachloroethane
[6] When reacted with compounds like cyclohexene, 2,2,4-trimethylpentl-ene, 1-hexene, 1-octene, 2-methyl-1-butene and 2,2,4-trimethyl-2-pentene, it yields allylic monobromides via bromination.
Dibromotetrachloroethane loses both of its bromine atoms, leaving tetrachloroethylene and hydrogen bromide.
[8] Malaguti exposed a mixture of Tetrachloroethylene (then known as chloréthose) and bromine to sunlight.
It was named Bromure de chloréthose ("bromide of chlorethose") after its synthesis method.
[5] Similar to Malaguti's method, modern synthesis of dibromotetrachloroethane uses bromine dissolved in carbon tetrachloride.