1,2-Wittig rearrangement
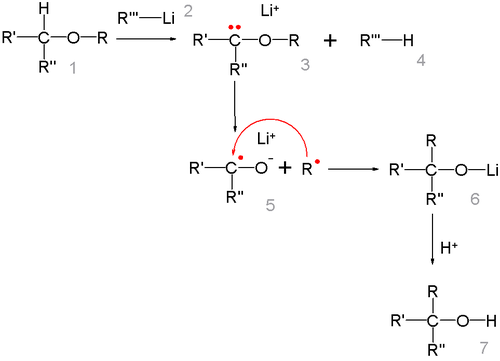
A 1,2-Wittig rearrangement is a categorization of chemical reactions in organic chemistry, and consists of a 1,2-rearrangement of an ether with an alkyllithium compound.
[1] The reaction is named for Nobel Prize winning chemist Georg Wittig.
The radical-ketyl pair is short lived and due to a solvent cage effect some isomerizations take place with retention of configuration.
[5] The reaction of allyl phenyl ether 1 with sec-butyllithium at −78 °C gives the lithiated intermediate 2 which on heating to −25 °C only shows the rearranged product 5 but not 4 after trapping the lithium alkoxide with trimethylsilyl chloride.
Additional evidence for this mechanism is provided by the finding that with a para tert-butyl substituent the reaction is retarded.