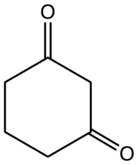
1,3-Cyclohexanedione
It is a colorless compound that occurs naturally.
The compound exists mainly as the enol tautomer.
[2] 1,3-Cyclohexanedione is produced by semi-hydrogenation of resorcinol:[3][4] 1,3-Cyclohexanedione exists in solution predominantly as the enol tautomer.
It reacts under acid catalysis with alcohols to 3-alkoxyenones.
Examples of commercial products include cycloxydim, clethodim, tralkoxydim, butroxydim, sethoxydim, profoxydim, and mesotrione.