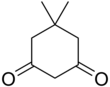
Dimedone
It is a white solid that is soluble in water, as well as ethanol and methanol.
It once was used as a reagent to test for the aldehyde functional group.
Dimedone is prepared from mesityl oxide and diethyl malonate via a Michael addition reaction.
[1][2] Dimedone is in equilibrium with its tautomer in solution — in a 2:1 keto to enol ratio in chloroform.
Crystalline dimedone contains chains of molecules, in the enol form, linked by hydrogen bonds:[4]