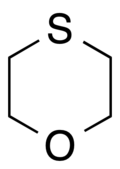
1,4-Oxathiane
Alternate ways are to dehydrate bis(hydroxy ethyl) sulfide by heating with potassium hydrogen sulfate.
[1] The original 1912 preparation of 1,4-oxathiane involved iodoethyl ether with potassium sulfide in alcohol.
[1] The sulfur atom in 1,4-oxathiane can undergo reaction as other substituted sulfides can.
It can be oxidised to a sulfoxide with calcium hypochlorite or sodium periodate,[2] or continuing to a sulfone.
Similarly iodine in acetic acid reacts to make 4-iodo-1,4-oxathianium iodide.