Hydrofluoric acid
It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon).
Many organofluorine compounds are prepared using HF as the fluorine source, including Teflon, fluoropolymers, fluorocarbons, and refrigerants such as freon.
[5] A 5% to 9% hydrofluoric acid gel is also commonly used to etch all ceramic dental restorations to improve bonding.
[5][7] Because of its ability to dissolve iron oxides as well as silica-based contaminants, hydrofluoric acid is used in pre-commissioning boilers that produce high-pressure steam.
The catalyst protonates the alkenes (propylene, butylene) to produce reactive carbocations, which alkylate isobutane.
Digestion of the mineral with sulfuric acid at elevated temperatures releases a mixture of gases, including hydrogen fluoride, which may be recovered.
[4] Because of its high reactivity toward glass, hydrofluoric acid is stored in fluorinated plastic (often PTFE) containers.
[19] At high concentrations, HF molecules undergo homoassociation to form polyatomic ions (such as bifluoride, HF−2) and protons, thus greatly increasing the acidity.
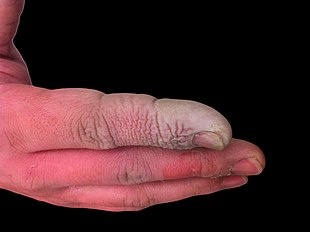
Symptoms of exposure to hydrofluoric acid may not be immediately evident, and this can provide false reassurance to victims, causing them to delay medical treatment.
[24] Symptoms of HF exposure include irritation of the eyes, skin, nose, and throat, eye and skin burns, rhinitis, bronchitis, pulmonary edema (fluid buildup in the lungs), and bone damage[25] due to HF strongly interacting with calcium in bones.
[26] In a concentrated form, HF can cause severe tissue destruction through lesions and mucous membrane damage, but dilute HF is still dangerous because of its high lipid affinity, leading to cellular death of nerves, blood vessels, tendons, bones, and other tissues.