Pentadiene
These pentadiene derivatives are susceptible to lipid peroxidation, far moreso than monounsaturated or saturated fatty acids.
The basis for this reactivity is the weakness of doubly allylic C-H bonds, leading to pentadienyl radicals.
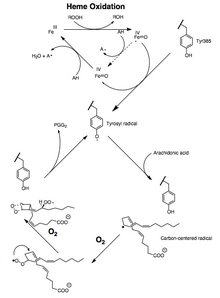
[8] Cyclooxygenases ("COX") are enzymes that generate prostanoids, including thromboxane and prostaglandins such as prostacyclin.
One practical consequence is that polyunsaturated fatty acids have poor shelf life owing to their tendency toward autoxidation, leading, in the case of edibles, to rancidification.
A number of complexes are known, including bis(pentadienyl) iron, Fe(C5H7)2, the "open" analog of ferrocene.