1-Fluoro-2,4-dinitrobenzene
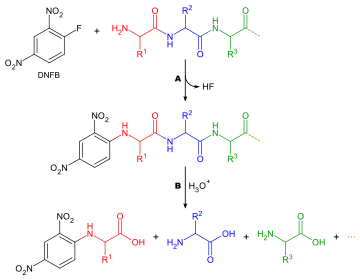
1-Fluoro-2,4-dinitrobenzene (commonly called Sanger's reagent, dinitrofluorobenzene, DNFB or FDNB) is a chemical that reacts with the N-terminal amino acid of polypeptides.
In 1936, Gottlieb presented a synthesis in which 1-chloro-2,4-dinitrobenzene reacted with potassium fluoride (KF) in nitrobenzene:[3] In 1945, Frederick Sanger described its use for determining the N-terminal amino acid in polypeptide chains, in particular insulin.
[4] Sanger's initial results suggested that insulin was a smaller molecule than previously estimated (molecular weight 12,000), and that it consisted of four chains (two ending in glycine and two ending in phenylalanine), with the chains cross-linked by disulfide bonds.
[5] Following Sanger's initial report of the reagent, the dinitrofluorobenzene method was widely adopted for studying proteins, until it was superseded by other reagents for terminal analysis (e.g., dansyl chloride and later aminopeptidases and carboxypeptidases) and other general methods for sequence determination (e.g., Edman degradation).
More recently, Sanger's reagent has also been used for the rather difficult analysis of distinguishing between the reduced and oxidized forms of glutathione and cysteine in biological systems in conjunction with HPLC.