Peptide synthesis
The chemical synthesis of peptides can be carried out using classical solution-phase techniques, although these have been replaced in most research and development settings by solid-phase methods (see below).
[2] Pioneered by Robert Bruce Merrifield,[4][5] SPPS allows the rapid assembly of a peptide chain through successive reactions of amino acid derivatives on a macroscopically insoluble solvent-swollen beaded resin support.
[2] Since the peptide remains covalently attached to the support throughout the synthesis, excess reagents and side products can be removed by washing and filtration.
This approach circumvents the comparatively time-consuming isolation of the product peptide from solution after each reaction step, which would be required when using conventional solution-phase synthesis.
At the end of the synthesis, the crude peptide is cleaved from the solid support while simultaneously removing all protecting groups using a reagent such as trifluoroacetic acid.
[2] The crude peptide can be precipitated from a non-polar solvent like diethyl ether in order to remove organic soluble byproducts.
For this reason so-called continuous chromatography processes such as MCSGP are increasingly being used in commercial settings to maximize the yield without sacrificing purity.
An important feature that has enabled the broad application of SPPS is the generation of extremely high yields in the coupling step.
The minimization of amino acid racemization during coupling is also of vital importance to avoid epimerization in the final peptide product.
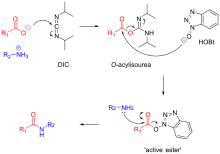
In addition to HBTU and HATU other common reagents include HCTU (6-ClHOBt), TCFH (chloride) and COMU (ethyl cyano(hydroxyimino)acetate).
[21] Solid supports for peptide synthesis are selected for physical stability, to permit the rapid filtration of liquids.
Suitable supports are inert to reagents and solvents used during SPPS and allow for the attachment of the first amino acid.
[22] Swelling is of great importance because peptide synthesis takes place inside the swollen pores of the solid support.
[citation needed] Two principle protecting group schemes are typically used in solid phase peptide synthesis: so-called Boc/benzyl and Fmoc/tert-butyl approaches.
Before the advent of SPPS, solution methods for chemical peptide synthesis relied on tert-butyloxycarbonyl (abbreviated 'Boc') as a temporary N-terminal α-amino protecting group.
[28] In addition, Boc/benzyl SPPS may be preferred over the Fmoc/tert-butyl approach when synthesizing peptides containing base-sensitive moieties (such as depsipeptides or thioester moeities), as treatment with base is required during the Fmoc deprotection step (see below).
[1] Final removal of the peptide from the solid support occurs simultaneously with side chain deprotection using anhydrous hydrogen fluoride via hydrolytic cleavage.
Scavengers such as cresol must be added to the HF in order to prevent reactive cations from generating undesired byproducts.
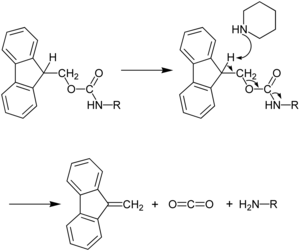
Because the liberated fluorenyl group is a chromophore, Fmoc deprotection can be monitored by UV absorbance of the reaction mixture, a strategy which is employed in automated peptide synthesizers.
The ability of the Fmoc group to be cleaved under relatively mild basic conditions while being stable to acid allows the use of side chain protecting groups such as Boc and tBu that can be removed in milder acidic final cleavage conditions (TFA) than those used for final cleavage in Boc/Bzl SPPS (HF).
[35] Hence, this became known as the Bergmann-Zervas synthesis, which was characterised "epoch-making" and helped establish synthetic peptide chemistry as a distinct field.
Thiol protecting groups used in peptide synthesis requiring later regioselective disulfide bond formation must possess multiple characteristics.
Thanks to inline analytics, such as UV/Vis spectroscopy and the use of Variable Bed Flow reactor (VBFR) that monitor the resin volume, on-resin aggregation can be identified and coupling efficiency can be evaluated.
Fragment condensation is better than stepwise elongation for synthesizing sophisticated long peptides, but its use must be restricted in order to protect against racemization.
The most common form of native chemical ligation uses a peptide thioester that reacts with a terminal cysteine residue.
[58] Other methods applicable for covalently linking polypeptides in aqueous solution include the use of split inteins,[59] spontaneous isopeptide bond formation[60] and sortase ligation.
[citation needed] The simple pre-sequence (e.g. Lysine (Lysn); Glutamic Acid (Glun); (LysGlu)n) that is incorporated at the C-terminus of the peptide to induce an alpha-helix-like structure.
This can potentially increase biological half-life, improve peptide stability and inhibit enzymatic degradation without altering pharmacological activity or profile of action.
[65][66] The strategy for the solid-phase synthesis of cyclic peptides is not limited to attachment through Asp, Glu or Lys side chains.
This has the disadvantages that the efficiencies of solid-phase synthesis are lost in the solution phase steps, that purification from by-products, reagents and unconverted material is required, and that undesired oligomers can be formed if macrocycle formation is involved.