11-Aminoundecanoic acid
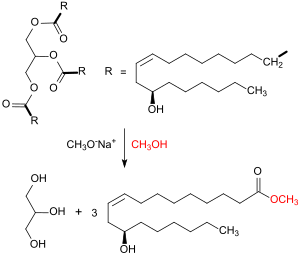
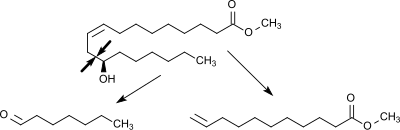
Methylricinoleate is evaporated at 250 °C, mixed with hot steam (600 °C) in a 1:1 ratio and decomposed in a cracking furnace at 400 - 575 °C at a retention time of about 10 seconds into its cleavage products heptanal and methyl undecenoate.
The cleavage of the aliphatic chain occurs in this variant of the steam cracking selectively between the hydroxymethylene and the allyl-methylene group.
The hydrolysis of the methyl ester with sodium hydroxide proceeds at 25 °C within 30 min with quantitative yield.
The undecenoic acid is dissolved in toluene and, in the presence of the radical initiator benzoyl peroxide (BPO), gaseous hydrogen bromide is added, in contrary to the Markovnikov rule ("anti-Markovnikov").
When the reaction is complete, water is added and the mixture is heated to 100 °C to remove the excess ammonia.
[5] N-acyl derivatives of 11-aminoundecanoic acid in the form of oligomeric amides have remarkable properties as gelling agents for water and organic solvents.