15-Crown-5
It is a cyclic pentamer of ethylene oxide that forms complex with various cations, including sodium (Na+)[2] and potassium (K+);[3] however, it is complementary to Na+ and thus has a higher selectivity for Na+ ions.
15-Crown-5 can be synthesized using a modified Williamson ether synthesis:[4] It also forms from the cyclic oligomerization of ethylene oxide in the presence of gaseous boron trifluoride.
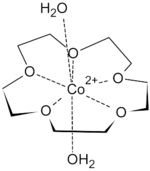
First-row transition metal dications fit snugly inside the cavity of 15-crown-5.
The binding of transition metal cations results in multiple hydrogen-bonded interactions from both equatorial and axial aqua ligands, such that highly crystalline solid-state supramolecular polymers can be isolated.
Seven coordinate species are most common for transition metal complexes of 15-crown-5, with the crown ether occupying the equatorial plane, along with 2 axial aqua ligands.