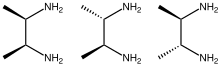
2,3-Butanediamine
Three stereoisomers exist, meso and a pair of enantiomers.
[4] Alternative, it is produced by reduction of dimethylglyoxime with lithium aluminium hydride.
[5] The meso and the d,l diastereomers can be separated by fractional crystallization of the hydrochlorides.
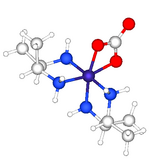
[6] In coordination chemistry, 2,3-butanediamine (abbreviated bn) has illuminates aspects of the stereochemistry.
The structure of [Co(meso-2,3-butanediamine)2CO3]+ confirms the presence of the rarely observed axial methyl groups on each of the diamine-cobalt rings.