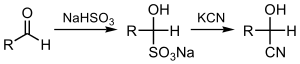
Cyanation
In organic synthesis, cyanation is the attachment or substitution of a cyanide group on various substrates.
Generally, KCN or its less toxic surrogate Zn(CN)2 are used as nucleophilic cyanide sources.
Catalytic cycles are believed to proceed through a standard Pd (0/II) pathway with reductive elimination forging the key C-C bond.
[7] Palladium catalysis conditions for aryl iodides, bromides, and even chlorides have been developed:[8] Nickel-catalyzed cyanations avoid the use of precious metals, and can take advantage of benzyl cyanide or acetonitrile as a cyanide source, via reductive C-C bond cleavage:[9] Sandmeyer cyanation is a means of converting aniline derivatives to benzonitriles.
These methods can proceed with or without transition metal mediation:[12] Radical approaches to arene C-H cyanation are known.