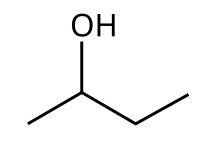
2-Butanol
This secondary alcohol is a nonflammable, colorless liquid that is soluble in three parts water and completely miscible with organic solvents.
[6] In the laboratory it can be prepared via Grignard reaction by reacting ethylmagnesium bromide with acetaldehyde in dried diethyl ether or tetrahydrofuran.
Although some butan-2-ol is used as a solute, it is mainly converted to butanone (methyl ethyl ketone, MEK), an important industrial solvent and found in many domestic cleaning agents and paint removers.
This widespread error originated because of Beilstein's Handbuch der Organischen Chemie (Handbook of Organic Chemistry).
As alcohols, unlike ethers, are not widely known to be capable of forming peroxide impurities, the danger is likely to be overlooked.