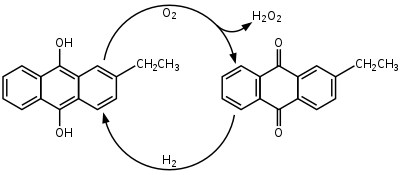
2-Ethylanthraquinone
This pale yellow solid is used in the industrial production of hydrogen peroxide (H2O2).
[1][2] 2-Ethylanthraquinone is prepared from the reaction of phthalic anhydride and ethylbenzene: Both phthalic anhydride and ethylbenzene are readily available, being otherwise used in the large-scale production of plastics.
The tetrahydro derivative of 2-alkylanthraquinone is easily hydrogenated but is more difficult to oxidize.
The formation of the tetrahydro derivative can be suppressed through the selection of catalysts, solvents, and reaction conditions.
Some suggested solvent mixtures are polyalkylated benzenes and alkyl phosphates or tetraalkyl ureas, trimethylbenzenes and alkylcyclohexanol esters, and methylnaphthalene and nonyl alcohols.