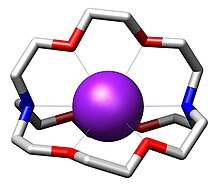
2.2.2-Cryptand
This bicyclic molecule is the most studied member of the cryptand family of chelating agents.
Their high affinity for alkali metal cations illustrates the advantages of "preorganization", a concept within the area of supramolecular chemistry.
For the design and synthesis of [2.2.2]cryptand,[3] Jean-Marie Lehn shared the Nobel Prize in Chemistry.
The compound was originally prepared starting with the diacylation of the diamine-diether:[4] The resulting macrocyclic diamide is reduced by lithium aluminium hydride.
The resulting macrocyclic diamine tetraether reacts with a second equivalent of [CH2OCH2COCl]2 to produce the macrobicyclic diamide.