Cryptand
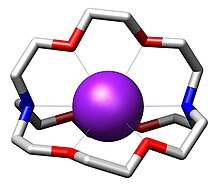
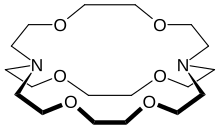
In chemistry, cryptands are a family of synthetic, bicyclic and polycyclic, multidentate ligands for a variety of cations.
[5] All-amine cryptands exhibit particularly high affinity for alkali metal cations, which has allowed the isolation of salts of K−.
This three-dimensional encapsulation mode confers some size-selectivity, enabling discrimination among alkali metal cations (e.g. Na+ vs. K+).
For example, addition of 2,2,2-cryptand to a solution of sodium in ammonia affords the salt [Na(2,2,2-crypt)]+e−, isolated a blue-black paramagnetic solid.
[13] Although rarely practical, cryptands can serve as phase transfer catalysts since their cationic complexes are lipophilic.