3,4-Dihydropyran
The six-membered C5O ring has the unsaturation adjacent to oxygen.
The isomeric 3,6-dihydropyran has a methylene separating the double bond and oxygen.
[1] Dihydropyran is prepared by the dehydration of tetrahydrofurfuryl alcohol over alumina at 300–400 °C.
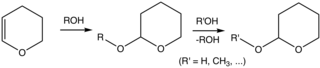
[3][4] Reaction of the alcohol with DHP forms a THP ether, protecting the alcohol from a variety of reactions.
The alcohol can later be restored by acidic hydrolysis, concomitant with formation of 5-hydroxypentanal.