Tetrahydropyran
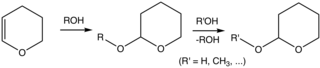
2-Tetrahydropyranyl (THP-) ethers derived from the reaction of alcohols and 3,4-dihydropyran are commonly used as protecting groups in organic synthesis.
[2] Furthermore, a tetrahydropyran ring system, i.e., five carbon atoms and an oxygen, is the core of pyranose sugars, such as glucose.
In gas phase, the THP exists in its lowest energy Cs symmetry chair conformation.
[3] One classic procedure for the organic synthesis of tetrahydropyran is by hydrogenation of the 3,4-isomer of dihydropyran with Raney nickel.
Oxanes are the class of hexic cyclic ether rings with tetrahydropyran as the root chemical.