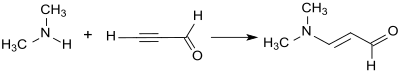
3-Dimethylaminoacrolein
[3] It is a stable chemical, unlike the parent compound 3-aminoacrolein [ru],[6] and can be used as a comparably nontoxic precursor for the genotoxic, mutagenic, and potentially carcinogenic malondialdehyde.
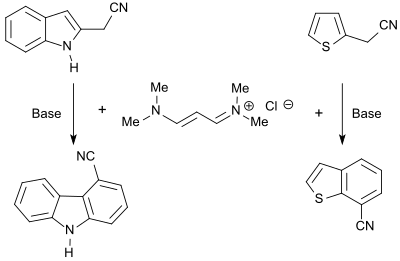
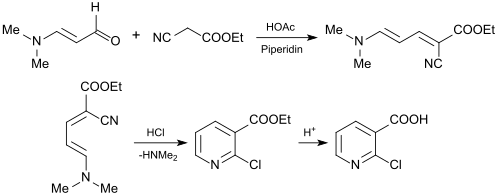
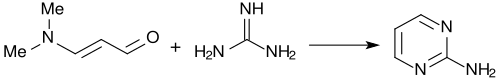
Therefore, 3-dimethylaminoacrolein and vinamidines derived there from (composed of vinylogous amidines) or vinamidinium salts (substituted 1,5-diazapentadienes)[8] can be used as reactive molecular building blocks for the formation of nitrogen-containing heterocycles, such as pyridines, pyrimidines, pyrroles or pyrazoles.
[11] They react with phosgene and dimethylformamide (which forms in-situ the Vilsmeier reagent) in 68% yield to 3-ethoxypropenylidene dimethylammonium chloride, an enol ether iminium salt.
[4] The amidine formed with 2-naphthylamine and the dimethyl sulfate adduct can be cyclized with sodium methoxide to give benzo[f]quinoline (1-azaphenanthrene).
[17][18] Occasionally, the iminium salt from the reaction of the Vilsmeier reagent and the vinyl ether (a precursor of 3-dimethylaminopropenal) is directly used for synthesis, e. g. for pyrazoles.
The salt reacts also with cyclopentadiene in the presence of sodium amide in liquid ammonia to give the aminofulvene derivative.
[22] Lactones (e.g. γ-butyrolactone) or cyclic ketones (such as cyclopentanone) form with the vinylamidinium salt of 3-dimethylaminoacrolein and dimethylamine hydrochloride the corresponding dienaminones in 91% and 88% yield.




![Synthese von Benzo[f]chinolin mit 3-Dimethylaminoacrolein](http://upload.wikimedia.org/wikipedia/commons/thumb/6/68/Synthese_von_1-Azaphenanthren.svg/400px-Synthese_von_1-Azaphenanthren.svg.png)