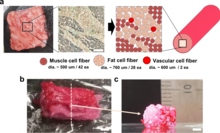
3D bioprinting
[1][2][3] Generally, 3D bioprinting uses a layer-by-layer method to deposit materials known as bio-inks to create tissue-like structures that are later used in various medical and tissue engineering fields.
Currently, bioprinting can be used to print tissue and organ models to help research drugs and potential treatments.
[7] Nonetheless, translation of bioprinted living cellular constructs into clinical application is met with several issues due to the complexity and cell number necessary to create functional organs.
Common technologies used for bioprinting are computed tomography (CT) and magnetic resonance imaging (MRI).
[14]: 165 In the second step, the liquid mixtures of cells, matrix, and nutrients known as bioinks are placed in a printer cartridge and deposited using the patients' medical scans.
3D bioprinting for fabricating biological constructs typically involves dispensing cells onto a biocompatible scaffold using a successive layer-by-layer approach to generate tissue-like three-dimensional structures.
[13] Bioreactors work in either providing convective nutrient transport, creating microgravity environments, changing the pressure causing solution to flow through the cells, or adding compression for dynamic or static loading.
[14]: 198 Researchers in the field have developed approaches to produce living organs that are constructed with the appropriate biological and mechanical properties.
This approach relies on the physical process of embryonic organ development as a model to replicate the tissues of interest.
[19] There is a "scaffold-free" model that uses self-assembling spheroids that subjects to fusion and cell arrangement to resemble evolving tissues.
Autonomous self-assembly depends on the cell as the fundamental driver of histogenesis, guiding the building blocks, structural and functional properties of these tissues.
[12] Bio-ink is a material made from living cells that behaves much like a liquid, allowing people to 'print' it in order to create the desired shape.
[22] Eccentric screw driven systems allow for a much more precise deposition of low to high viscosity materials due to the self-sealing chambers in the extruder.
[26] The bioink itself for this approach can be a blend of polymer hydrogels, naturally derived materials such as collagen, and live cells suspended in the solution.
[27] The development of tubular structures has found the layered extrusion achieved via these techniques desirable for the radial variability in material characterization that it can offer, as the coaxial nozzle provides an inner and outer tube for bioink flow.
[29] Photo-polymerization techniques rather use photoinitiated reactions to solidify the ink, moving the beam path of a laser to induce the formation of a desired construct.
In this form of printing, plastic residues are melted down and individual layered in sections to create a desired shape.
Droplet-based bioprinting is a technique in which the bioink blend of cells and/or hydrogels are placed in droplets in precise positions.
[33] Thermal technologies use short duration signals to heat the bioink, inducing the formation of small bubbles which are ejected.
A significant aspect of the study of droplet-based approaches to bioprinting is accounting for mechanical and thermal stress cells within the bioink experience near the nozzle-tip as they are extruded.
They are composed of living cells and enzymatic supplements to nurture an environment that supports the biological needs of the printed tissue.
[34] Cell-encapsualting hydrogels are used in extrusion based bioprinting methods, while gelatin MethacryloylGelatin methacrylon (GelMA) and acellular comprised bioinks are most often used in tissue engineering techniques that require cross-linkage and precise structural integrity.
The precursor to the adoption of 3D printing in healthcare was a series of trials conducted by researchers at Boston Children's Hospital.
The trials were a success as the patients remained in good health 7 years after implantation, which led a research fellow named Anthony Atala, MD, to search for ways to automate the process.
[42] In 2022, the first success of a clinical trial for a 3D bioprinted transplant that is made from the patient's own cells, an external ear to treat microtia,[43] was reported.
[44] 3D bioprinting contributes to significant advances in the medical field of tissue engineering by allowing for research to be done on innovative materials called biomaterials.
Some of the most notable bioengineered substances are usually stronger than the average bodily materials, including soft tissue and bone.
[45][46] This technology provides an alternative to natural meat harvesting methods if the livestock industry is plagued by disease.
[51] 3D bioprinting allows functional microorganisms to be placed in structures that provide mechanical stability and protects them from environmental conditions.