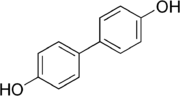
4,4'-Biphenol
It is primarily used in the production of polymers, particularly liquid crystals where it imparts high thermal stability.
[2][3] As the direct oxidative coupling of phenol gives a mixture of isomers,[4][5] 4,4′-biphenol is instead prepared from 2,6-di-tert-butylphenol, where para-coupling is the only possibility.
[3] A reaction with oxygen produces phenol-radicals which undergo rapid dimerisation, ultimately forming a diphenoquinone.
[2] In the final step, high temperature dealkylation is performed to remove the butyl groups, producing the desired 4,4′-biphenol product.
[3] If groups less bulky that t-butyl are used then polyphenylene ethers such as poly(p-phenylene oxide) can be produced.