Sublimation (phase transition)
Notable examples include sublimation of dry ice at room temperature and atmospheric pressure, and that of solid iodine with heating.
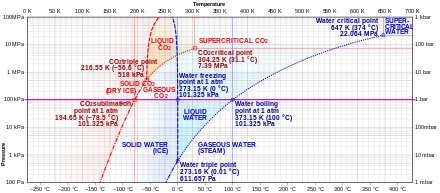
Sublimation is caused by the absorption of heat which provides enough energy for some molecules to overcome the attractive forces of their neighbors and escape into the vapor phase.
The words "gradual" and "rapid" have acquired special meanings in this context and no longer describe the rate of sublimation.
[citation needed] The term sublimation refers specifically to a physical change of state and is not used to describe the transformation of a solid to a gas in a chemical reaction.
Similarly the combustion of candles, containing paraffin wax, to carbon dioxide and water vapor is not sublimation but a chemical reaction with oxygen.
[7] In freeze-drying, the material to be dehydrated is frozen and its water is allowed to sublime under reduced pressure or vacuum.
[8] Naphthalene, an organic compound commonly found in pesticides such as mothballs, sublimes easily because it is made of non-polar molecules that are held together only by van der Waals intermolecular forces.
[10] At low temperature, its vapour pressure is high enough, 1 mmHg at 53 °C,[11] to make the solid form of naphthalene evaporate into gas.
Iodine sublimes gradually and produces visible fumes on gentle heating at standard atmospheric temperature.
Under this reduced pressure, the solid volatilizes and condenses as a purified compound on a cooled surface (cold finger), leaving a non-volatile residue of impurities behind.
It was mentioned by alchemical authors such as Basil Valentine and George Ripley, and in the Rosarium philosophorum, as a process necessary for the completion of the magnum opus.
Here, the word sublimation was used to describe an exchange of "bodies" and "spirits" similar to laboratory phase transition between solids and gases.
Valentine, in his Le char triomphal de l'antimoine (Triumphal Chariot of Antimony, published 1646) made a comparison to spagyrics in which a vegetable sublimation can be used to separate the spirits in wine and beer.
From the equipartition theorem gaseous rotation and translation contribute 1.5RT each to the final state, therefore a +3RT correction.
The process uses the science of sublimation, in which heat and pressure are applied to a solid, turning it into a gas through an endothermic reaction without passing through the liquid phase.
[citation needed] In order to transfer the image from the paper to the substrate, it requires a heat press process that is a combination of time, temperature and pressure.
[citation needed] The result of the sublimation process is a nearly permanent, high resolution, full color print.